Copper(I) Chloride CAS 7758-89-6 Cuprous Chloride Purity ≥99.95%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Copper(I) Chloride or Cuprous Chloride (CAS: 7758-89-6) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Copper(I) Chloride |
| Synonyms | Cuprous Chloride; Copper Monochloride |
| CAS Number | 7758-89-6 |
| CAT Number | RF-PI2076 |
| Stock Status | In Stock, Production Capacity 500MT/Month |
| Molecular Formula | CuCl |
| Molecular Weight | 99.00 |
| Melting Point | 430℃(lit.) |
| Boiling Point | 1490℃(lit.) |
| Density | 4.140 g/cm3 (25℃) |
| Sensitivity | Light Sensitive. Hygroscopic. Air Sensitive |
| Solubility in Water | Sparingly Soluble in Water |
| Solubility | Very Soluble in Concentrated HCl. Soluble in Ammonium Hydroxide. Insoluble in Ethanol and Acetone. |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Grey or Light Green Powder or Crystals |
| Purity / Analysis Method | ≥99.95% (Based On Trace Metals Analysis) |
| Total Metallic Impurities | 0~500 ppm |
| Copper (Cu) | 62.9~65.5% (Complexometric EDTA) |
| Iron (Fe) | ≤0.002% |
| Arsenic (As) | ≤0.0005% |
| Substances Not Precipitated by Hydrogen Sulfide | ≤0.15% |
| Sulfate (SO4) | ≤0.05% |
| Insoluble Matter (in Acid) | ≤0.01% |
| Calcium (Ca) | ≤0.01% |
| Potassium (K) | ≤0.02% |
| Sodium (Na) | ≤0.05% |
| Lead (Pb) | ≤0.02% |
| ICP | Confirms Copper Component Conforms |
| X-Ray Diffraction | Conforms to Structure |
| Acid Solution | Transparent |
| Test Standard | Enterprise Standard |
Package: 25kg/bag, 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Copper(I) Chloride, also known as Cuprous Chloride (CAS: 7758-89-6) is used as catalyst for organic reactions; catalyst, decolorizer and desulfuring agent in petroleum industry; in denitration of cellulose; as condensing agent for soaps, fats and oils; in gas analysis to absorb carbon monoxide. Contact with strong acids forms monovalent copper salts and toxic hydrogen chloride gas. Forms shock-sensitive and explosive compounds with potassium, sodium, sodium hypobromite, nitromethane, acetylene. Keep away from moisture and alkali metals. Attacks metals in the presence of moisture. Reacts with moist air to form cupric chloride dihydrate. May attack some metals, paints, and coatings. May be able to ignite combustible materials.
-

Copper(I) Chloride CAS 7758-89-6 Cuprous Chlori...
-

Copper(I) Bromide Cuprous Bromide CAS 7787-70-4...
-

Copper(I) Iodide CAS 7681-65-4 Purity >99.0% (C...
-
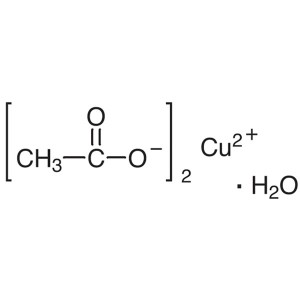
Copper(II) Acetate Monohydrate CAS 6046-93-1 Pu...
-
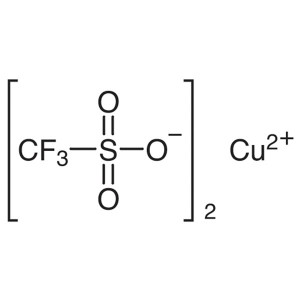
Copper(II) Trifluoromethanesulfonate CAS 34946-...
-
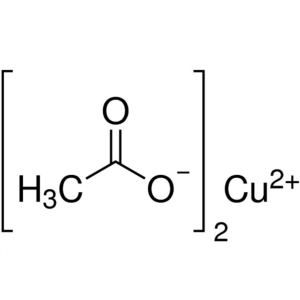
Cupric Acetate Anhydrous CAS 142-71-2 Purity >9...
-
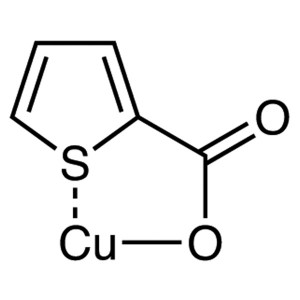
Copper(I) thiophene-2-carboxylate CAS 68986-76-...
-

Sodium Thiosulfate CAS 7772-98-7 Purity >99.0% ...
-
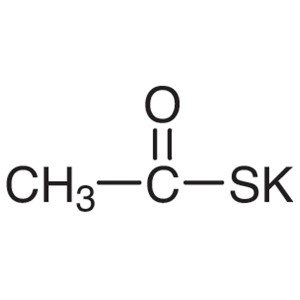
Potassium Thioacetate CAS 10387-40-3 Purity >98...
-

Ferrous Sulfate Heptahydrate CAS 7782-63-0 Assa...
-

Cerium(III) Chloride Heptahydrate CAS 18618-55-...
-

Ferrous Sulfate Monohydrate CAS 13463-43-9 Puri...
-
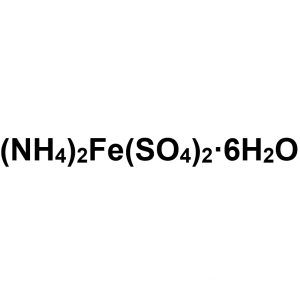
Ammonium Iron(II) Sulfate Hexahydrate CAS 7783-...
-
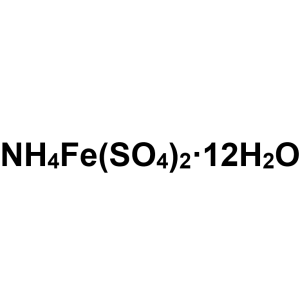
Ammonium Iron(III) Sulfate Dodecahydrate CAS 77...
-
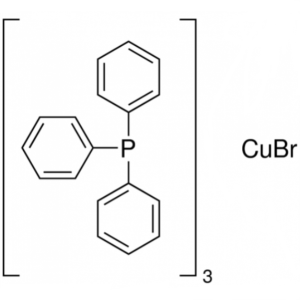
Bromotris(triphenylphosphine)copper(I) CAS 1570...
-

Copper(II) Sulfate Anhydrous CAS 7758-98-7 Puri...

